The Nghiem Lab is actively involved in several ongoing research projects focused on the biology, treatment and prevention of skin cancers. Investigators conduct both basic research to clarify the mechanisms causing a disease, and translational research to transform their research findings into clinical solutions. View a list of current research projects below.
For inquiries about PhD, graduate or post-doctoral research opportunities in the Nghiem Lab, please email Dr. Paul Nghiem at pnghiem@uw.edu.
For general inquiries, please email mccteam@uw.edu.
Merkel Cell Carcinoma (MCC)
Our laboratory focuses primarily on Merkel cell carcinoma (MCC), a rare but lethal skin cancer. In about 80% of cases, MCC is caused by the Merkel cell polyomavirus, while the remaining 20% of cases are caused by damage from UV light (sunlight).
In 2010, our team found that the immune system plays a very important role in determining how well MCC patients do. Specifically, patients with a robust immune response (CD8 T cells) within the tumor were far more likely to beat their cancer after standard treatment. Based on these findings, our lab has worked to develop immune-based therapies to effectively treat MCC. In 2017, based on studies our team led, immune therapy replaced chemotherapy as the standard of care for MCC patients that cannot be treated with local therapy.
Our team maintains a clinical database of over 1600 patients with MCC, accumulated since 2004. This data informs our clinical studies and trials, and the corresponding clinical samples (blood, tumor tissues, etc) are used to study the immunobiology of this cancer. This database has allowed us to develop a blood test for antibodies to the Merkel virus that accurately tracks disease recurrences and is increasingly used nationwide. A ‘circulating tumor DNA test’ is now being studied that will work on both virus-negative as well as virus-positive patients. Current interests of the lab include mechanisms by which MCC avoids the immune system, identification of approaches to increase anti-cancer immunity, and new ways to help radiation activate an immune response against cancer.
Our laboratory has created an informative website on this cancer aimed for patients, their families, and physicians that has over 80,000 visitors per year: merkelcell.org.
Click infographics to enlarge.
Major Accomplishments
- A clinical trial of avelumab (Bavencio; anti-PD-L1) in MCC patients with advanced, chemotherapy-refractory MCC showed a response rate of 33% with 74% of responses lasting greater than one year (Kaufman et al, Lancet Oncology, 2016). This trial led to the FDA approval of Avelumab for MCC in 2017.
- Pembrolizumab (Keytruda; anti-PD-1) in MCC patients with systemic disease had an objective response rate of 56% with progression free survival at 6 months of 67% (Nghiem et al, New England Journal of Medicine, 2016). This study led to the NCCN designating this type of immune drug as a preferred therapy for patients with advanced MCC, and FDA approval of peembrolizumab in 2018.
- An immune stimulating drug called G100 (a TLR4 agonist) led to increased CD4 and CD8 T cell infiltration into tumors and led to responses in 4/10 patients (Bhatia et al, Clinical Cancer Research, 2018).
Translational Biology Projects
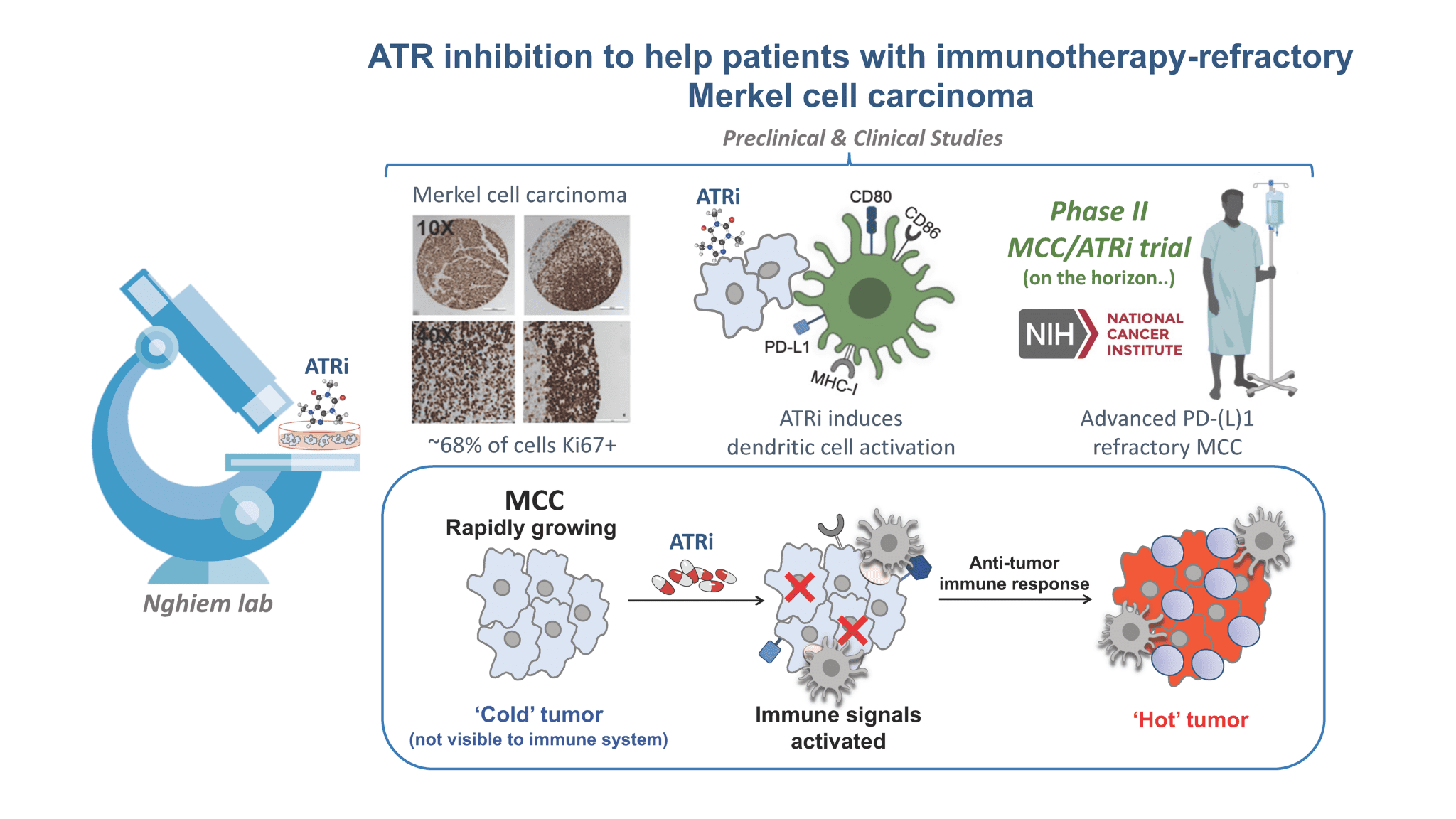
DNA Damage Response Inhibitor (ATR Inhibitor)
Our focus is to develop treatments for the ~50% of MCC patients who need more than current immunotherapies. New drugs that prevent cancer cells from responding normally to DNA damage may also stimulate an anti-tumor immune response. An ATR inhibitor kills cancer cells by stopping them from responding normally to DNA damage. New data suggest this approach stimulates an anti-tumor immune response. We are designing a clinical trial to determine if this therapy is safe, shrinks MCC tumors, and remains effective over the long term. Click here view an animation of how ATR works.
Therapeutic Vaccine to Prevent Recurrence
After initial surgery and/or radiation removes all evident cancer, we currently have no treatments to lower the chance of MCC recurrence, even though we know the risk of recurrence is more than 20% in virtually all patients. We have developed a therapeutic vaccine that we hope will help the immune system recognize and destroy remaining cancer cells in the body, for patients who have virus-positive MCC. As was apparent in the development of COVID-19 vaccines, it is advantageous to have several options to compare, so we are actively exploring three different types of vaccines for the Merkel virus. The first of these agents began clinical testing in July 2022.
Clinical Research Projects
Personalized Risk Calculator
To help MCC physicians and patients make informed decisions about surveillance (observing patients while they are at risk for their cancer recurring), our group developed a patient-specific recurrence risk calculator. The calculator allows patients to enter their date of diagnosis, sex, age at diagnosis, site of their primary tumor and immune suppression status to then view their recurrence risk. Because recurrence risk falls by more than 90% by 3 years after diagnosis, the calculator soon provides reassurance and encourages appropriate tapering of visits and scans for patients who do not have a recurrence. The calculator uses data published in 2022 (McEvoy, et al, JAMA Dermatology). An additional publication is in preparation. View the current version of this calculator at merkelcell.org/recur.
Circulating Tumor DNA (ctDNA)
This test detects DNA released by Merkel cell carcinoma (MCC) tumor cells into the blood. The presence and quantity of MCC tumor-specific DNA mutations in a patient’s blood appears to accurately reflect residual or recurrent MCC. This blood test works for both virus-positive and virus-negative patients to monitor response to treatment or to detect early recurrence. The test is currently being closely followed in a study involving six academic sites across the US.